Professor Cao Dianxue of the College of Materials Science and Chemical Engineering of HEU published a Solvent Induced Activation Reaction of MoS2 Nanosheets within Nitrogen/Sulfur-Codoped Carbon Network Boosting Sodium Ion Storage in the international journal Small. It made a breakthrough in sodium ion battery, which improved the ratio performance and cycle life of MoS2 electrode material by regulating the electrolyte components.
Renewable energy technology can effectively reduce carbon emissions and assist achieve carbon neutrality, and battery energy storage technology is the key to make up for the intermittent shortcomings of renewable energy. Sodium-ion batteries (SIBs) have become one of the promising alternative chemical power sources for large scale energy storage system on account of the their low cost and abundant sources of sodium. However, the large radius (10.2 Å) is not conducive to the rapid transfer and storage of sodium ions. MoS2, as a classical two-dimensional material, becomes a capable anode candidate for sodium-ion batteries. However, MoS2 presents a disparate electrochemical performance in the ether-based and ester-based electrolyte with unclear mechanism. Moreover, the application of MoS2 is hindered by its low rate characteristics and poor cycling stability, which is ascribed to the unsatisfied electron conductivity and volume expansion under repeatedly sodiation/desodiation. Therefore, it is necessary to combine nanostructure, composite design, and electrolyte regulation strategies to further improve its electrochemical performance.
Recently, Prof. Cao and his group reported the fabrication and sodium ion storage behavior of a tiny MoS2 nanosheets embedded in nitrogen/sulfur-codoped carbon (MoS2@NSC) networks, which was published in Small. They found the ether-based electrolyte lead to a unique capacity growth of MoS2@NSC in the original stage of cycling. But in the ester-based electrolyte, MoS2@NSC shows a usual capacity decay. The increasing capacity attributes to the gradual transformation from MoS2 to MoS3 with the structure reconstruction. Based on the above mechanism, MoS2@NSC demonstrates an excellent recyclability and the specific capacity keeps around 286 mAh g−1 at 5 A g−1 after 5000 cycles with an ultra-low capacity fading rate of only 0.0034 % per cycle. Moreover, the MoS2@NSC composite exhibits the high capacities of 292 mA h g−1 at 30 A g−1. In addition, a MoS2@NSC‖Na3V2(PO4)3 full cell with ether-based electrolyte is assembled and demonstrates a capacity of 71 mAh g−1, suggesting the potential application of MoS2@NSC. This work reveals the electrochemical conversion mechanism of MoS2 in the ether-based electrolyte and highlights significance of the electrolyte design on the promoting Na+ storage behavior.
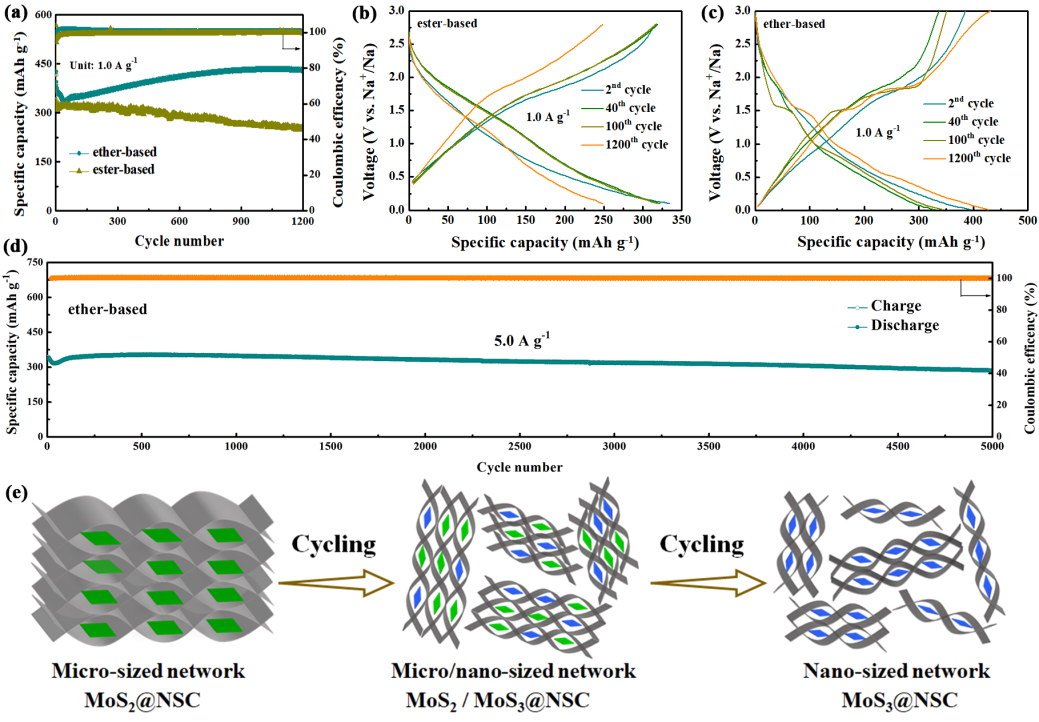
Figure 1. (a) Cycle performance, (b, c) GCD curves, (d) long-term cycle performance of MoS2@NSC composites in ether-based and/or ester-based electrolyte; (d) Schematic illustration of the morphology and composition evolution of the MoS2@NSC electrode before and after cycling in ether-based electrolyte.
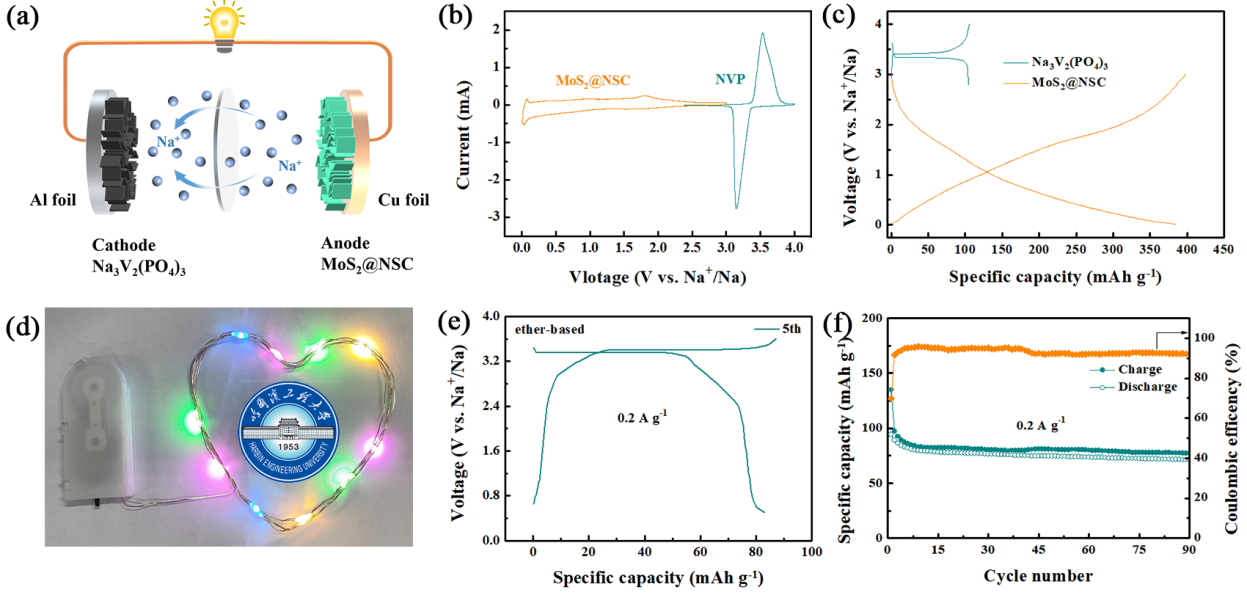
Fig. 2 (a) Schematic illustration of the SIFC for discharging process, (b) CV curves of MoS2@NSC and Na3V2(PO4)3 at 0.5 mV s−1, (c) GCD profiles of MoS2@NSC and Na3V2(PO4)3 at 0.1 A g−1, (d) The photo of lighting LED powered by two SIFC, (e) GCD profiles of SIFC and (f) Cycle properties of SIFC.
